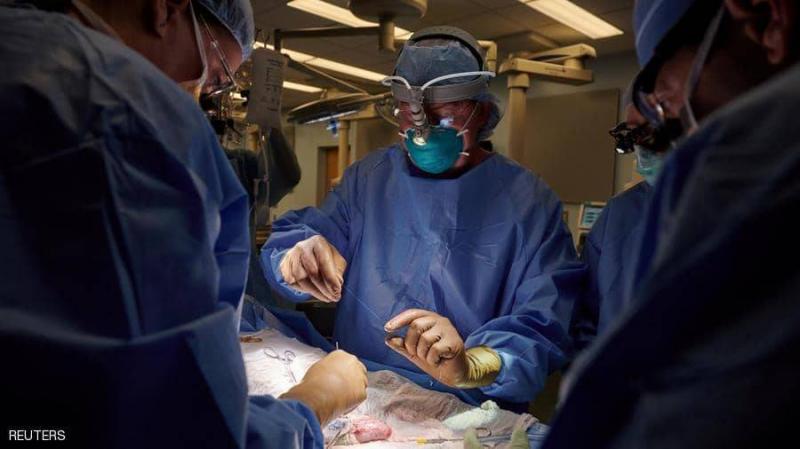
American surgeons have successfully performed the first transplant of a pig kidney into a human body without the recipient's immune system rejecting the transplanted organ. This is a significant advancement that could potentially alleviate the severe shortage of available human organs for transplantation. The surgical procedure took place at NYU Langone Medical Center and involved a pig whose genes were modified so that its tissues did not contain a molecule known to likely cause immediate rejection of the transplanted organ.
Researchers told Reuters that the kidney recipient was a brain-dead patient who showed signs of impaired kidney function, and her family consented to the experiment before she was removed from life support. For three days, the new kidney was connected to the patient's blood vessels and was kept outside her body, allowing researchers to handle it directly.
Robert Montgomery, the lead surgeon on the transplant, said the results of the kidney function tests "looked largely normal." He added that the kidney produced "the amount of urine you would expect" from a transplanted human kidney, and there was no evidence of the strong early rejection seen with unmodified pig kidneys transplanted into non-human primates. Montgomery noted that the abnormal creatinine level in the recipient, an indicator of impaired kidney function, returned to normal after the transplant.
Currently, nearly 107,000 people in the United States are awaiting organ transplants, including over 90,000 waiting for kidney transplants, according to the United Network for Organ Sharing. The average waiting time for a kidney is between three to five years.
For decades, researchers have been working on the possibility of using animal organs for transplants, but the challenge has been preventing the human body from immediately rejecting the transplanted organ. Montgomery's team hypothesized that eliminating the pig gene responsible for a carbohydrate causing rejection would prevent the issue. This gene is a sugar molecule called "alpha-gal."
The genetically modified pig, named "GalSafe," was developed by Revivicor, a subsidiary of United Therapeutics Corp, and was approved by the U.S. Food and Drug Administration in December 2020 for use as food for those with meat allergies and as a potential source for human therapies. The FDA has stated that medical products developed from pigs will still require specific approval from them before use in humans.