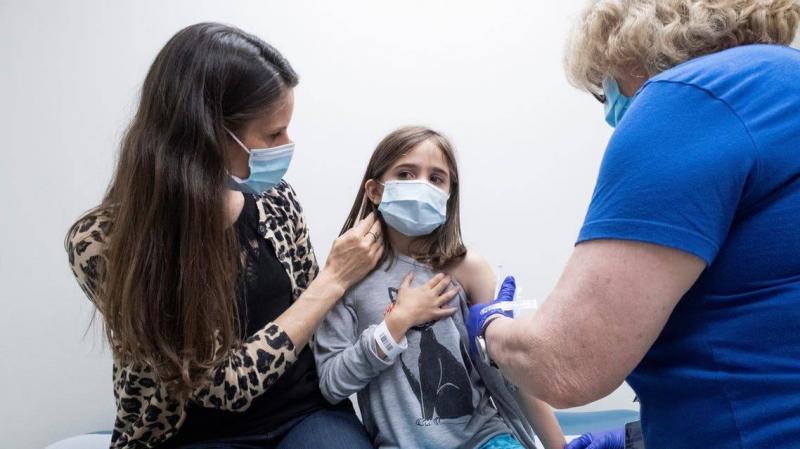
The U.S. Food and Drug Administration has authorized the use of the Pfizer-BioNTech vaccine for children aged 12 to 15, with the Centers for Disease Control and Prevention expected to recommend expanded eligibility later today, Wednesday. Some vaccination sites began immunizing children against Covid-19 on Tuesday morning, a day after the FDA approved the Pfizer vaccine for this age group. Jacob Laney, 14, was standing in line at a vaccination site in Georgia early Tuesday, hoping to receive the vaccine. He told CNN, "My friend got Covid-19, and it looked really bad, and I don’t want to get it either." He added, "Once I get two doses of the Pfizer vaccine, I think I will feel less scared of getting sick and having health problems due to Covid-19."
Cameron Carrion, also 14, expressed his satisfaction at receiving the vaccine dose. He said, "I feel it’s better to get it because then I can go out more and not just stay at home doing nothing." The vaccinations are somewhat premature, as doctors were not technically supposed to start administering the vaccine to this age group until the CDC recommended doing so. The Advisory Committee on Immunization Practices (ACIP), an advisory panel for the CDC, is scheduled to hold an emergency meeting Wednesday to discuss and vote on the matter early in the afternoon. This advice will then go to CDC Director Dr. Rochelle Walensky, who is very likely to give the green light within hours, according to CNN.
However, doctors already have the vaccine on hand, and CDC approval is a foregone conclusion. This area of medical practice is regulated by the states, but since the vaccine is already licensed, it is difficult to prevent medical professionals from exercising their own judgment. The FDA issued an emergency use authorization for the Pfizer vaccine for younger age groups after a clinical trial involving 2,260 children aged 12 to 15 showed that the vaccine was 100% effective and well-tolerated.