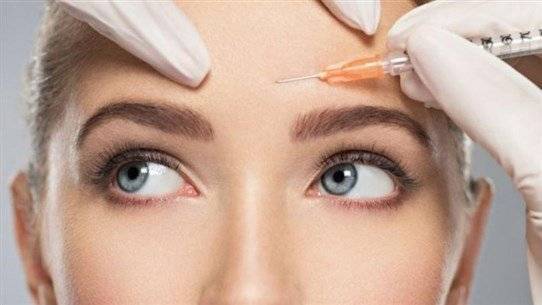
The Food and Drug Administration (FDA) has approved a new injectable anti-wrinkle treatment that lasts about two months longer than Botox for use in adults. This marks the first approval of its kind in over a decade. Daxxify, manufactured by Revance, is injected into facial lines and around the eyes, lasting for approximately six months, surpassing Botox's three to four months duration. Mark Foley, the CEO of the Tennessee-based biotechnology company, mentioned that they are already investigating whether it can also be used to treat lip spasms and cervical dystonia—an involuntary contraction of neck muscles.
Daxxify works in the same way as Botox, acting as a neuromuscular blocker that effectively freezes muscles, helping to prevent wrinkles. Its developers hope to encourage more people to switch to this treatment as it requires fewer injections each year. Botox customers typically need at least three doses annually, while Daxxify could reduce this to two with the same treatment effects.
The price of the new treatment has not been disclosed, but its main competitor usually costs between $250 and $500 per injection in each treatment area. Foley stated that they plan to initially roll it out in a few pharmacies, the locations of which have not been revealed, for use among select patients, before it becomes commercially available.
Daxxify is the first treatment to use peptides to stabilize the formula, rather than the animal or human proteins typically used. The FDA reported that the treatment was approved for adult use in an innovative drug list published this week, based on phase 3 trials involving 2,700 patients and over 4,200 injections.
Results showed that 74% of participants experienced improvement in their lines four weeks after treatment, with effects lasting an average of six months—some patients reported that it was still effective after nine months. The most common side effect was headache (experienced by six percent of participants), followed by eyelid droop (2%). No one experienced muscle weakness or breathing difficulties, but the FDA warned that these could occur with the treatment.
Foley stated that Daxxify will be a "pioneering innovation" and represents the culmination of "years of leading research and development." The product had been awaiting approval for a long time, with its initial review postponed in November 2020 due to the COVID pandemic.