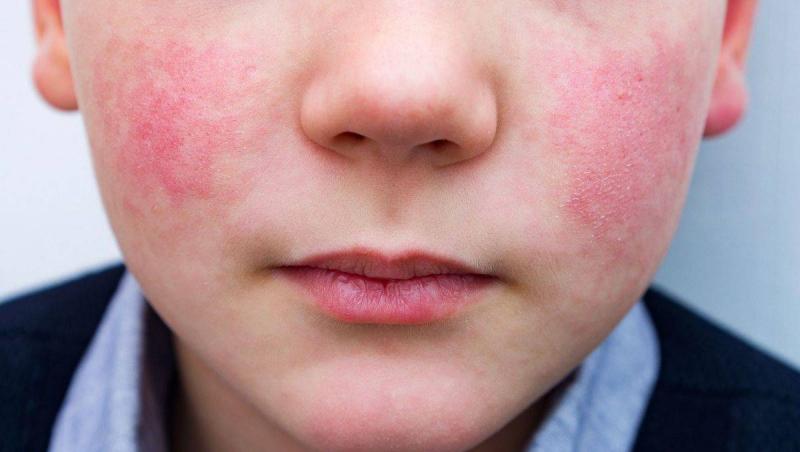
The outbreak of an infectious virus in the Beqaa region has led to the temporary closure of one of the prestigious schools in Zahle, denying students from attending as the school's administration decided to shut its doors for one day due to the virus's rapid spread. Concerns are rising from Beqaa to Beirut and all over Lebanon regarding the potential spread of this infection. In this context, Dr. Nidal Hassan, the head of the Veterinarians' Syndicate, confirmed in a statement that Parvo Virus B19 does not transmit from animals to humans and does not cause any symptoms in humans, stating that there is no need for panic. Dr. Hassan pointed out that the symptoms of Parvo in animals differ from those affecting humans, and he confirmed the existence of many other strains of this virus.
What is this disease? Parvovirus B19, or simply B19, is the first known human parvovirus and has been recognized since 2005. It belongs to the family of small viruses, specifically the genus of erythroparvoviruses. The name "parvo" is derived from Latin, meaning "small," reflecting its classification as one of the smallest DNA viruses, measuring only 23-26 nanometers in diameter. Parvovirus B19 is primarily known for its infectious nature in children but can also affect adults. It is traditionally known as the cause of a childhood rash commonly referred to as "fifth disease" or "slapped cheek syndrome."
The virus was accidentally discovered in 1975 by Australian virologist Yvonne Cossart and got its name "B19" because it was identified in tube B19 during a large series of calibration plates.
**Transmission**
The virus is mainly spread through respiratory droplets from the infected person; however, cases of transmission via blood have been reported. The secondary infection risk for people exposed to the virus at home is about 50%, and roughly half of this proportion applies to classmates.
**Infection**
Symptoms begin six days after exposure to the virus (ranging from 4 to 28 days, with an average of 16 to 17 days) and last about a week. Patients with normal immunity can transmit the infection before they show symptoms, but the appearance of symptoms likely halts further transmission. Individuals with antibodies against Parvovirus B19 are generally protected from reinfection, though reinfection can occur in a few cases. About half of adults are immune to B19 due to previous infections.
**Epidemiology**
A significant increase in the number of cases is noted every three to four years, with 1998 being the last epidemic year for this virus. The virus particularly spreads in nurseries and schools. Human infections occur solely with Parvovirus B19; humans are not affected by parvoviruses specific to cats and dogs. No vaccine is currently available for Parvovirus B19, despite attempts being made to develop one.
**Treatment**
Currently, no treatments targeting Parvovirus B19 directly are available. Intravenous immunoglobulin (IVIG) has been a commonly considered alternative therapy, allowing doctors to administer it without halting chemotherapy drugs like Mel-ASCT. Additionally, this treatment has rare side effects; complications occurred in only 4 out of 133 patients, with two patients suffering acute kidney injury and two others experiencing pulmonary edema, despite 69 of the patients having transplant histories and 39 being HIV positive. This treatment has shown significant improvement compared to treatment with Rituximab. It has been shown that monoclonal antibodies against protein CD20 can cause acute hepatitis and neutropenia due to the activation of Parvovirus B19 or the enhancement of existing infections. However, it is important to note that IVIG therapy is not ideal, as 34% of the patients treated with it experienced relapse after 4 months.