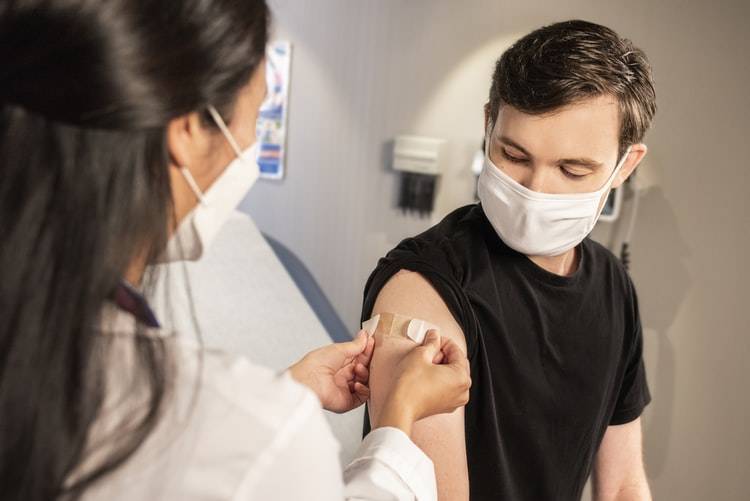
Since the beginning of the pandemic, scientists have been striving to better understand immunity to the novel coronavirus. For example, how long does a person remain immune after being infected with COVID-19 or receiving a vaccination? What might long-term immunity mean for booster vaccine doses? It is still too early to know, but experts are getting closer to solving the puzzle.
Dr. Peter Marks, director of the Center for Biologics Evaluation and Research at the U.S. Food and Drug Administration, stated during a webinar on the COVID-19 vaccine education project and equity that current information regarding potential coronavirus vaccine boosters suggests that they may be needed at some point, but it is unclear when that will be. Experts confirm that anyone who is currently fully vaccinated will remain protected. However, the uncertainty regarding the timeline for potential boosters stems from the fact that scientists still need time to collect data on how long immunity against COVID-19 might last in the future and how to address future variants of the virus.
When a person has "immunity" in general, it means they are protected from disease. Active immunity can be acquired either through vaccination or infection. The immune system develops antibodies either as a result of vaccination or in response to infection, and the immune response can maintain "memory." Immunity is often measured by the presence of antibodies, which are proteins produced by the immune system to help combat infections in the blood, and can usually be detected through laboratory testing. However, immune responses encompass much more than just antibodies; they also include B cells that produce antibodies and T cells that target infected cells.
Research has shown that both antibodies and T cells can recognize infections from different types of pathogens, such as the strains of the novel coronavirus circulating in the world today. Despite key differences that might make them spread more easily, they have enough similarities for the immune system's memory to recognize them. If someone has recovered from a previous infection and has natural immunity, vaccines can help boost their immune memory.
Currently, three coronavirus vaccines have been granted emergency use authorization in the United States: the Pfizer/BioNTech vaccine with two doses for ages 12 and older, the Moderna vaccine with two doses for ages 18 and older, and the Johnson & Johnson vaccine with a single dose for ages 18 and older. The three companies are investigating the potential use of boosters. Vaccine manufacturers are studying whether the immunity generated by these vaccines may wane over long periods, for example, after a year or more, and whether they also protect against variants of the coronavirus that may emerge and evolve. If this is the case, vaccinated individuals may need a booster dose of the vaccine to remain protected from the original coronavirus strain and newly emerging variants, somewhat like the recommendation for a tetanus booster every 10 years or the recommendation to receive different influenza vaccines each year.
For other viruses, typically a single episode of measles leaves a person immune for life. The same was true for smallpox before the virus was eradicated in the 1970s through a global vaccination campaign. Proper vaccination against measles and smallpox completely prevents infection. However, respiratory viruses like influenza and coronavirus are more challenging; people can contract influenza repeatedly, and influenza vaccines generally provide only partial protection against infections and severe diseases, due to the multiple influenza viruses that spread due to mutations.
Nevertheless, the coronavirus has a slower mutation rate than influenza. Still, doctors are concerned that coronavirus may become like influenza, requiring a new vaccine every year because prevalent strains change rapidly and vaccine-induced immunity wanes quickly. In the case of coronavirus vaccines, numerous studies have evaluated the immune responses triggered by the Moderna and Pfizer vaccines against the original strain of the virus, compared to the variants. According to the Centers for Disease Control and Prevention, these studies have observed modest or non-existent deficiencies in the cellular immune recognition of variants. Thus, cellular immunity may help reduce disease severity in infections caused by variants that partially evade neutralizing antibodies.
It is difficult to predict how decreased neutralizing activity will impact the efficacy of the COVID-19 vaccine, but studies indicate that the neutralizing antibody activity observed among fully vaccinated individuals was generally higher than that observed among those who recovered from COVID-19. Marks from the FDA mentioned in April that data from clinical trials suggest that the protection provided by the Pfizer and Moderna vaccines against coronavirus is likely to last at least 9 months. However, experts have made an effort to emphasize that this does not mean immunity stops at 9 months. This duration represents the longest time volunteers in trials were monitored to assess their immunity and gather data. Immunity might last longer, and researchers just need time to evaluate it.
Dr. Amish Adalja, a senior researcher at the Johns Hopkins Center for Health Security who did not participate in the studies, stated in an email to CNN on Thursday that the medical community still needs data to determine the degree of waning immunity over time. This degree can be measured based on whether fully vaccinated individuals eventually experience breakthrough infections at a higher rate or have severe infections requiring hospitalization. Meanwhile, studies on natural immunity from previous coronavirus infections have taken somewhat longer than vaccine trials.